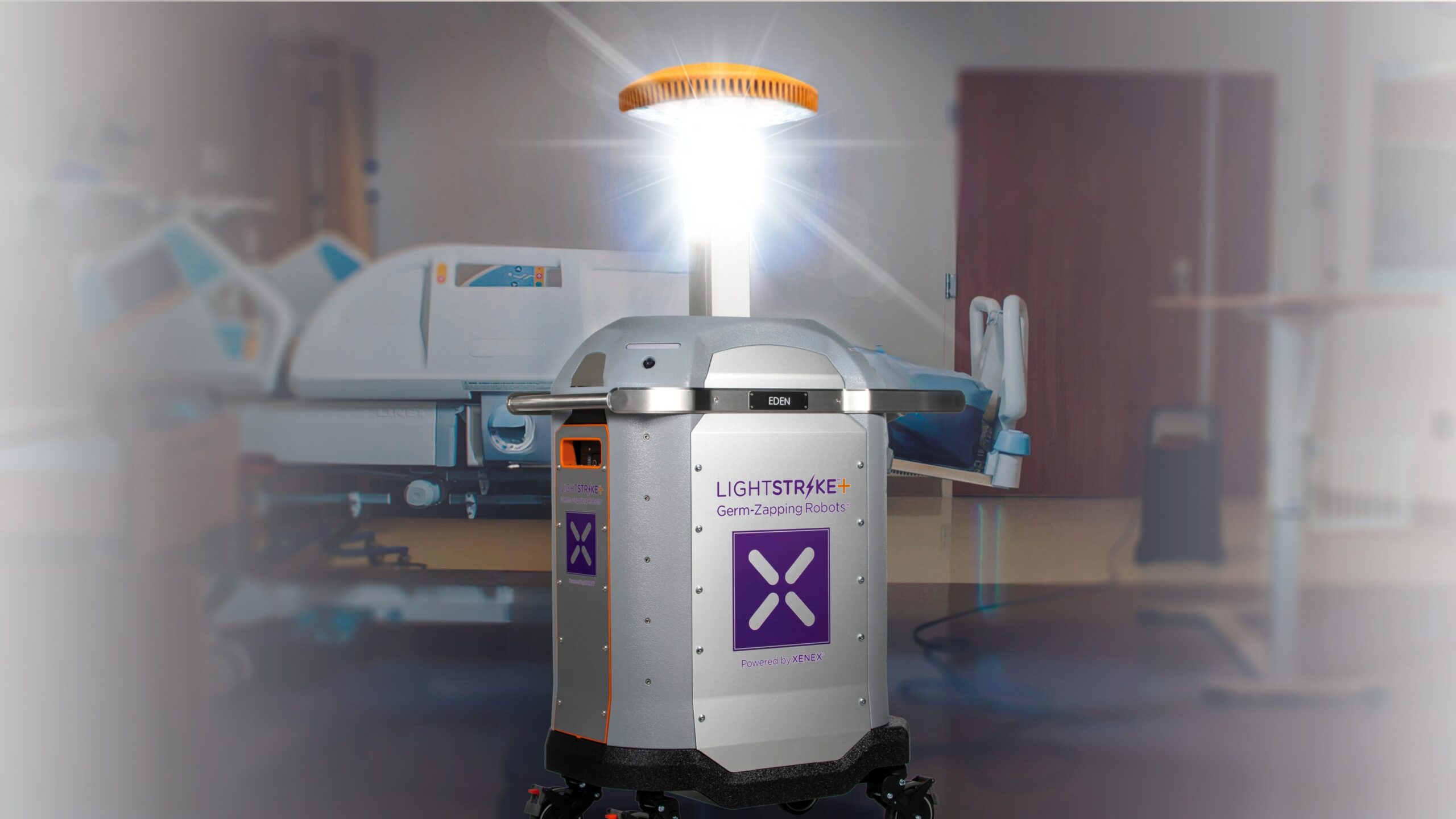
SAN ANTONIO–(BUSINESS WIRE)–The U.S. Food and Drug Administration (“FDA”) has granted Xenex Disinfection Services, Inc. (“Xenex”) De Novo authorization for the company’s LightStrikeTM+ device, a high-intensity, broad-spectrum ultraviolet (UV) light robot. The authorization creates a new medical device product classification under which the LightStrike+ robot is the first and only product of its type, setting the precedent for FDA regulation of UV robots intended for use in reducing pathogens on non-porous, high-touch surfaces in the healthcare environment.
















